A single-step, plasma-enhanced catalytic process to convert sulfur dioxide to pure sulfur from tail gas streams may provide a promising, more environmentally-friendly alternative to current multistage thermal, catalytic and absorptive processes, according to scientists at Penn State.
"Sulfur dioxides can cause significant environmental problems like acid rain, and it can cause sea acidification," said Xiaoxing Wang, associate research professor at the Penn State EMS Energy Institute. "Sulfur can also contribute to fine particulate matter in the air we breathe, which can be more severe than the sulfur dioxide itself."
Exposure to particulate matter was estimated to cause 4.2 million premature deaths and more than 100 million disability-adjusted life years—which measures years lost due to illness, disability or death—according to the Lancet Global Burden of Diseases Study, published in 2015.
According to Wang, current desulfurization methods can successfully remove sulfur dioxide from tail gas streams but not without significant drawbacks.
Flue gas desulfurization technologies, for example, are the most used methods to capture sulfur dioxide, but these processes create a large amount of solid waste in the form of metal sulfate that requires disposal. Furthermore, these processes produce wastewater that requires additional treatment, making the overall method costly and environmentally unfriendly.
Alternatively, sulfur dioxide can be reduced to solid elemental sulfur through catalysis—a chemical reaction brought on by a catalyst and usually a reducing agent such as hydrogen, methane, or carbon monoxide—and then used as a raw material for such things as fertilizer. However, high temperatures are normally needed in the traditional catalytic process to attain high conversion levels. This is not ideal because it uses a great deal of energy and there is a loss of catalyst activity, according to the scientists.
Due to these flaws, Wang and his colleagues tested a novel technology, a one-step, low-temperature plasma-assisted catalytic process that eliminates the need for high temperatures and creates far less waste than FGD technologies.
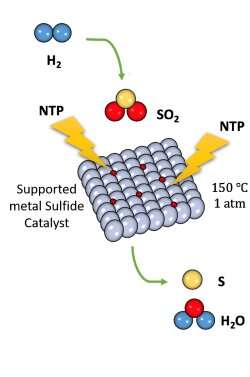
To test this process, the team loaded an iron sulfide catalyst into a packed bed reactor. Then they introduced the hydrogen and the sulfur dioxide gas mixtures, which passed through the catalyst bed at roughly 300 degrees Fahrenheit. They then turned on the nonthermal plasma and the reactions immediately began to occur.
Once the process completed, they analyzed the samples to see how much sulfur dioxide was in the gas and how much hydrogen was consumed. They also collected and analyzed the solid sulfur, which accumulates at the bottom of the reactor. They published their results in ACS Catalysis and a recent issue of the Journal of Catalysis.
"The temperature we used, 150 degrees C (about 300 degrees F), is higher than the sulfur melt point to avoid sulfur deposition over the catalyst," Wang said. "Through this process, the catalyst shows very excellent stability. When run for several hours, we do not see any deactivation. The activity and the selectivity stay the same."
The researchers also found that this process dramatically promoted sulfur dioxide reduction at low temperatures, enhancing conversion by 148% to 200 percent and 87 to 120 percent using hydrogen and methane, respectively.
Sean Knecht, assistant teaching professor in the School of Engineering Design, Technology and Professional Programs, said that NTP works because highly energetic electrons interact with gas molecules to produce reactive species—radicals, ions and excited molecules—enabling various chemical reactions at low temperature.
"The result is that the electrons are able to initiate what would appear to be thermodynamically-unfavorable chemical reactions through dissociation and excitation of reactants at much lower temperatures than thermal catalysis," Knecht said. "If these reactions can be undertaken at much lower temperatures than are typical for thermal catalysis, as we have shown, then the power input to future systems is significantly reduced, which is a big deal."
Wang added that using plasma allows them to achieve optimal performance using just 10 watts of electricity. Another advantage is that renewable energy, such as wind or solar, can be easily applied to this process to supply power to the plasma.
The researchers now want to better understand exactly how the plasma contributes to the catalysis process and seek to develop an even more effective catalyst for the process.
"A current challenge that we are working to address is further isolating the effects of the plasma versus effects of the catalyst and the synergistic aspects," Knecht said. "We are looking at some surface spectroscopy options presently and at some point, combining with computational modeling. Bringing these together can provide a more holistic understanding of the physics and chemistry at play."
If the process is commercializable, it has the potential to largely replace the current FDG technologies.
"It's highly beneficial to energy and the environment," Wang said. "Our process saves energy, reduces waste and saves water. This is very transformational."

