A RUDN University chemist has synthesized fluorescent compounds with "merry-go-round" molecules that can be used to create economical displays with organic LEDs (OLEDs). The nucleus of these molecules is a triangle of silver or copper atoms, and organic elements are bound to it through phosphorus atoms that rotate around them. This molecular geometry may allow researchers to create more efficient OLED screens. The article is published in Inorganic Chemistry.
Displays with OLEDs differ from other modern types of display such as plasma and LCD displays. OLEDs have higher brightness, contrast and lower power consumption. However, they are more expensive, and the raw material for their production—conductive polymers—is toxic, creating difficulties in production and disposal.
To reduce the cost of OLED displays and replace toxic raw materials, it is possible to use fluorescent complex compounds—molecules with small organic fragments surrounding the central ion of the metal instead of polymers. But to date, there are no complexes that show a clear advantage in brightness and efficiency over polymers. Sufficiently effective compounds based on iridium or platinum are expensive, and cheaper complexes with transition metal ions are not effective.
RUDN University chemist Alexander Smol'yakov has now discovered compounds to make OLED displays much brighter and more economical than polymer ones. The centers of these complexes are not platinum or iridium, but cheaper copper and silver, which also proved to be more effective and less toxic compared to polymers.
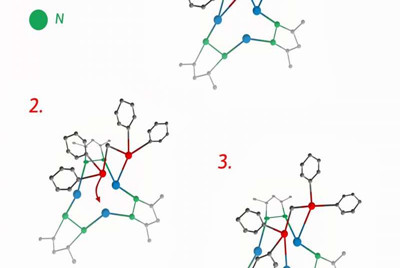
Smol'yakov synthesized a molecule in the center of which are three ions of monovalent copper or silver. To strengthen this structure, he stabilized it using derivatives of pyrazole—aromatic molecules with two nitrogen atoms in the cycle. He used organophosphorus molecules as ligands—electron donors surrounding ion. In this case, the ions of monovalent copper and silver form a three-center nucleus in the form of a triangle, and ligands join the nucleus through phosphorus atoms and remain quite mobile.
At room temperature, the energy of thermal oscillations is sufficient to break the bond
between phosphorus and metal for a short time. However, there are two phosphorus atoms
in a molecule, and there are three metal atoms. So one of the metal
atoms is always without a pair, and if there is a single phosphorus, the
metal atom attracts it immediately—that is, the ligand "jumps" to the
neighboring ion in the three-center nucleus and forms a bond that can be
broken via thermal fluctuations.
The molecule thus turns into a kind of molecular "merry-go-round." This configuration makes stable complexes with nuclei of silver ions, and complexes with nuclei of monovalent copper—the compounds do not decay immediately after synthesis, like many other structures of this type.
Chemists have found that such a "merry-go-round" structure of complex compounds leads to the emergence of two energy states, the transition between which can lead to luminescence. In the case of copper, this structure has a significant quantum yield—that is, the ratio of the number of absorbed and emitted photons is 41 percent.
Thus, researchers have for the first time managed to show a sufficiently high quantum yield on systems, which opens up new opportunities for novel OLED displays. The study was conducted jointly with scientists from INEOS RAS and Saint Petersburg State University.

